Our team members come from companies that have been pioneers in the implementation of Quality Standards in the biotech and pharmaceutical industry. We are specialized in the development of Advanced Therapies and Innovative Medicines and we have been responsible for the fine tuning of the first processes in Spain for the manufacturing of Gene and Cell Therapy medicines for clinical trials.
Company
Leading consulting firm in the field. Professionals with more than 20 years of experience in helping our clients to develop new medicines that will improve people’s lives.
Gradocell is a client-centered company. In addition to providing our own services, we work with our clients and offer them, upon request, one-off collaboration with other companies with expertise in each of the project’s strategic areas: Engineering (for the design and set-up of facilities); CMOs for third-party manufacturing; CROs for the management of clinical trials; service companies (cleaning and maintenance of cleanrooms, etc.), etc. Because of Gradocell’s comprehensive consulting services, all of our clients can focus on their main objectives, because we offer them all the resources they need


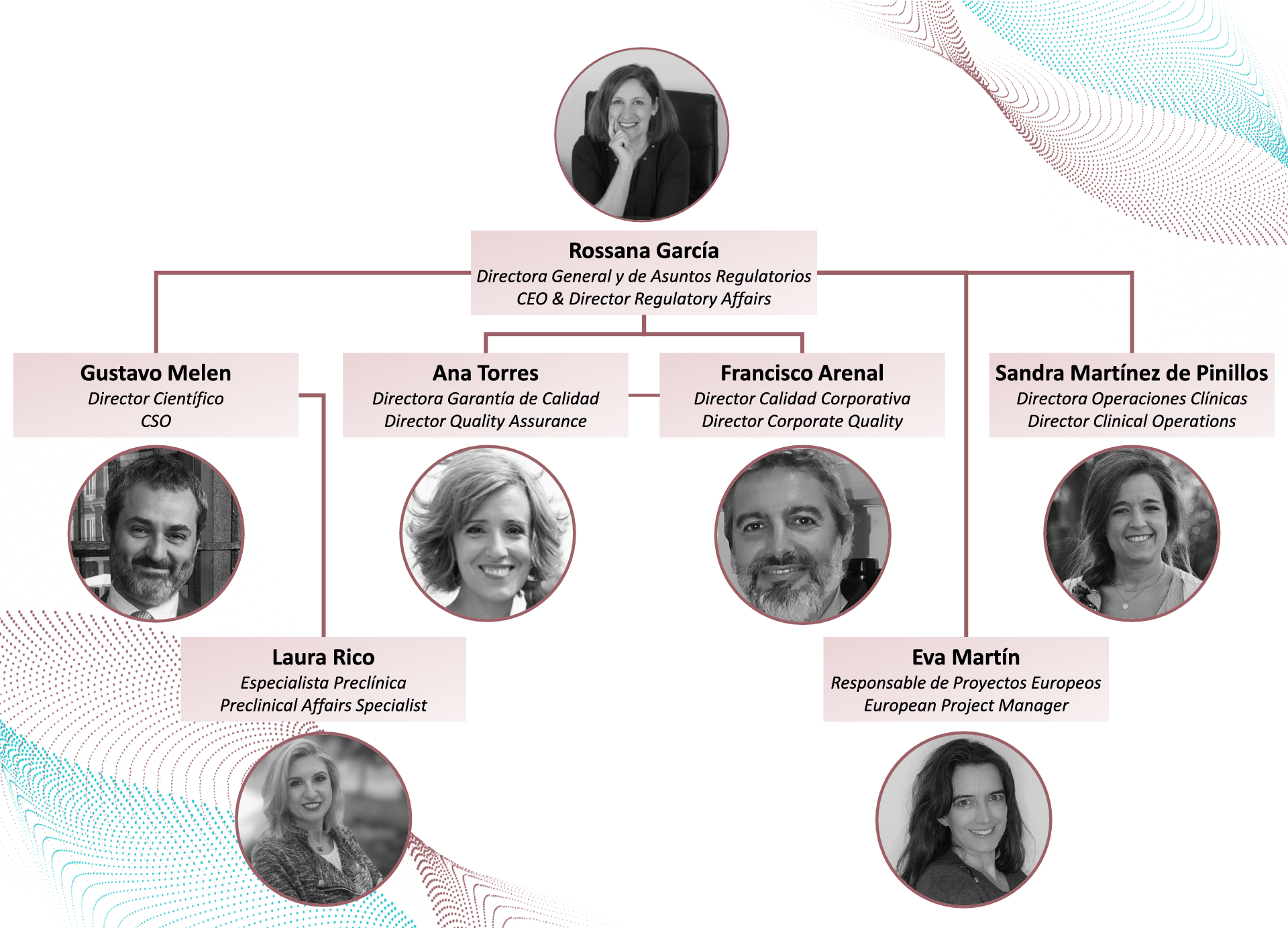
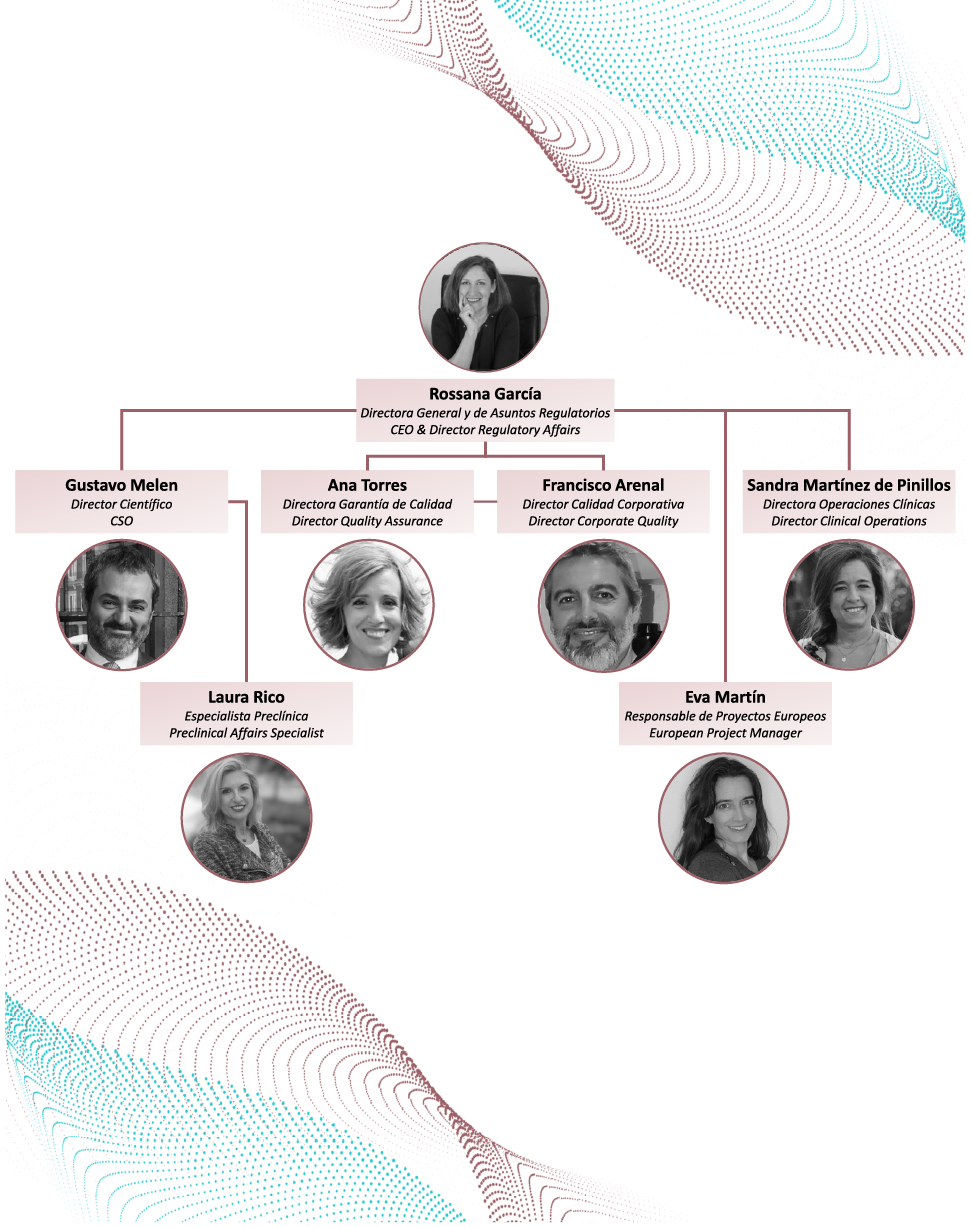
Meet the team!
Our team is composed of highly-qualified professionals, pioneers and experts in the design of regulatory strategy, manufacturing, clinical trial approvals, and the marketing and authorization of medicines used for Advanced Therapies (Cell Therapy, Gene Therapy and Tissue Engineering) and Innovative Medicines.